Enhance Liver Health
In a peer-reviewed human study on 85 participants with liver stress, the effectiveness of Siliphos® as a beneficial
approach for promoting a healthy liver was examined. The study aimed to evaluate the impact of Siliphos® administration on liver enzyme levels and other relevant markers.
The results revealed that Siliphos® administration had a positive effect on liver health indicators, independently of the individual's body mass index (BMI). Notably,Siliphos® optimized insulinemia, alanine aminotransferase (ALT), and γ-glutamyl transpeptidase (γ-GT) levels.
These findings suggest that Siliphos® could be considered an effective intervention for improving liver function in cases of liver stress. [1,2]
Significantly Optimized Bioavailability
These findings highlight the superior bioavailability of silybin achieved through the use of Siliphos®. The Phytosome formulation enables rapid absorption and sustained plasma levels of silybin. [3,4]
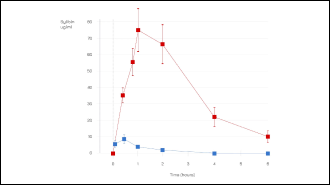
Figure 1: Plasma silybin profile after Siliphos® (upper line)and simple silybin (lower line) administration.
[1] Loguercio, C., Federico, A., Trappoliere, M., Tuccillo, C., de Sio, I., Di Leva, A., Niosi, M., D'Auria, M. V., Capasso, R., Del Vecchio Blanco, C., & Real Sud Group. (2007). The effect of a silybin-vitamin e-phospholipid complex on nonalcoholic fatty liver disease: A pilot study. Digestive Diseases and Sciences, 52(9), 2387-2395.
[2] Vailati, A., et al. (1993). Randomized open study of the dose-effect relationship of a short course of IdB 1016 in patients with viral or alcoholic hepatitis. Fitoterapia, 64, 219.
[3] Morazzoni, P., Magistretti, M. J., Giachetti, C., & Zanolo, G. (1992). Comparative bioavailability of Silipide, a new flavanolignan complex, in rats. European Journal of Drug Metabolism and Pharmacokinetics, 17, 39-44.
[4] Morazzoni, P., Montalbetti, A., Malandrino, S., & Pifferi, G. (1993). Comparative pharmacokinetics of silipide and silymarin in rats. European Journal of Drug Metabolism and Pharmacokinetics, 18, 289-297.